Oncology
Together, let’s overcome cancer !

SOLID TUMOUR – i-NanoT
The i-NanoT project is a multidisciplinary public–private consortium in Bourgogne-Franche-Comté designed to build a full nanomedicine value chain, from fundamental research to preclinical production. Its objective is to develop theranostic nanovectors (i.e. nanoparticles serving both diagnostic and therapeutic purposes) for a variety of pathologies such as cancers, infectious diseases, and inflammatory disorders.
Within this framework, the CGFL occupies a key position: as a cancer care centre and research hospital, it is one of the two clinical partners in the programme (the other being the local university hospital, CHU Dijon). As a clinical- and imaging-capable institution, CGFL provides access to advanced preclinical imaging and radiotherapy research teams that are essential for the biological evaluation (in vitro and in vivo) of nanovector candidates.
Specifically, CGFL is responsible for translational validation of nanovectors, conducting studies on biodistribution, toxicity, therapeutic efficacy potential, and preclinical modelling, prerequisites for any later clinical development phases. Moreover, CGFL contributes to the scientific and administrative governance of the project, coordinating between academic laboratories, industrial partners, and clinical stakeholders to ensure coherence and integration across development stages.
Beyond CGFL’s contribution, the i-NanoT consortium brings together academic laboratories (CB, ICMUB, INSERM and other labs in chemistry, biology, materials science, biophysics), industrial partners producing nanoparticles (SON SAS), and technology transfer/valorisation actors. The intended nanovectors encompass both mineral-based nanoparticles (e.g. gold, iron, silica) and organic forms (liposomes, virus-like particles), with production targeted at the “kg-lab” scale. This requires rigorous attention to reproducibility, quality control, scalability, and standardization — essential steps toward pre-industrial manufacturing.
The project officially spans 2024–2028 and is supported by a total budget of approximately €15.6 million funded by the European regional development programme (FEDER / FSE+).
Thanks to CGFL’s involvement, i-NanoT integrates fundamental research, technological development, clinical infrastructure, and translational capacity, creating a vital synergy needed to realize the promises of nanomedicine.
Consequently, i-NanoT represents a regional (and potentially national or European) ambition: to establish Bourgogne-Franche-Comté as a nanomedicine hub, ensuring a strong link between innovation, industry, and healthcare, with CGFL functioning as the strategic pivot anchoring the clinical and translational dimension of the initiative.

SOLID TUMOUR – COMETE
The COMETE project (moleCular radiOtherapy for METastatic colorectal and gastric cancErs) is an ambitious collaborative initiative supported by the European Regional Development Fund (FEDER) under the Bourgogne-Franche-Comté / Massif du Jura 2021–2027 programme. It brings together a Dijon-based consortium of three partners: the Centre Georges François Leclerc (CGFL), Oncodesign Precision Medicine (OPM), and the Institut de Chimie Moléculaire de l’Université de Bourgogne (ICMUB – CNRS UMR 6302).
The aim of COMETE is to develop a portfolio of radiopharmaceutical molecules—through the approach of Targeted Radionuclide Therapy (TRT) capable of selectively targeting tumour cells in metastatic digestive cancers (colon, stomach, and pancreas), enabling their destruction from within while minimising off-target toxicity.
To achieve this, the consortium has defined four major scientific workstreams:
- Identification and validation of novel tumour targets
- Development of TRT vectors and candidate radiopharmaceuticals
- Preclinical evaluation of therapeutic efficacy and toxicity
- Design of diagnostic companions: associated imaging agents to identify patients likely to benefit from treatment and to monitor tumour evolution over time
Funded by the FEDER for €7.8 million, out of a total projected budget of €9.2 million, the resources are allocated over five years between the three partners: €3.7 million for CGFL, €2.1 million for OPM, and €2.0 million for ICMUB.
Within the consortium, the CGFL plays a central and strategic role: supported by its IMATHERA nuclear medicine and imaging team, it will conduct preclinical validation of drug candidates including dosimetry, efficacy, and toxicity and pave the way for potential clinical trials. Through its implementation, COMETE could provide a new personalised therapeutic option for patients with metastatic digestive cancers who currently have limited or suboptimal treatment alternatives, while positioning Dijon as a centre of excellence in advanced targeted radionuclide therapy.
In summary, COMETE exemplifies the strength of Europe-supported collaboration between academic research, biotechnology, and clinical practice to advance vectorised radiotherapy with the prospect of significantly improving management and prognosis in advanced digestive cancers.
Associated publication:
Newsletter September 2025 : click here
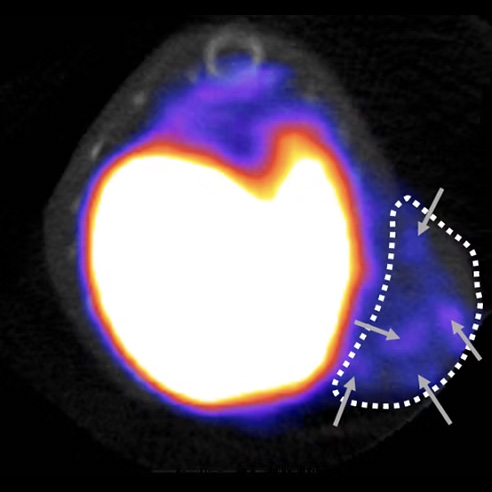
SPECT imaging of HER2 expression

SOLID TUMOUR – HER2-low BREAST CANCER
Breast cancer is the most common cancer in the world. In fact, breast tumours in women alone account for around 11.7% of new cases of cancer each year, with over two million women diagnosed in 2020, making this disease a major public health problem. At the molecular level, this malignant tumour is highly heterogeneous and can be divided into five groups (Luminal A, Luminal B HER2 negative, Luminal B HER2 positive, HER2 enriched, triple negative) based on the immunohistochemical expression of oestrogen receptors, progesterone receptors and human epidermal growth factor receptor 2 (HER2). Approximately 20% of newly diagnosed breast cancer cases are HER2-positive, and the prognosis for this group has improved with monoclonal antibody-based anti-HER2 targeted therapy. Despite this progress, a high percentage of patients (40-50%) have low HER2 expression (HER2-low) for whom anti-HER2 therapies are of little benefit. However, recent studies have shown that HER2-low patients could benefit from new therapies targeting HER2 based on ‘armed’ antibodies (Antibody drug conjugates, ADC). However, resistance to these new therapies has been observed. In addition, accurate determination of HER2 expression is essential to identify patients who could benefit from these therapies, particularly when the current reference tests for determining HER2-low/HER2-negative expression have been challenged for their poor accuracy. These data have led us to hypothesise that the production of theranostic probes (combining properties for diagnosis and therapy) targeting HER2, in a HER2-low context, could represent an innovative approach to overcome the current problems and thus improve the diagnosis and therapeutic management of these patients.
Our preliminary results have shown that nuclear imaging, using a HER2-specific probe, can detect the level of HER2 expression in various preclinical models of breast cancer. These results have enabled our platform to obtain ANR funding (EITHER2BC) of €324,000 over 3 years (2024-2027) to develop these probes for the diagnosis and therapy of HER2-low breast cancer.
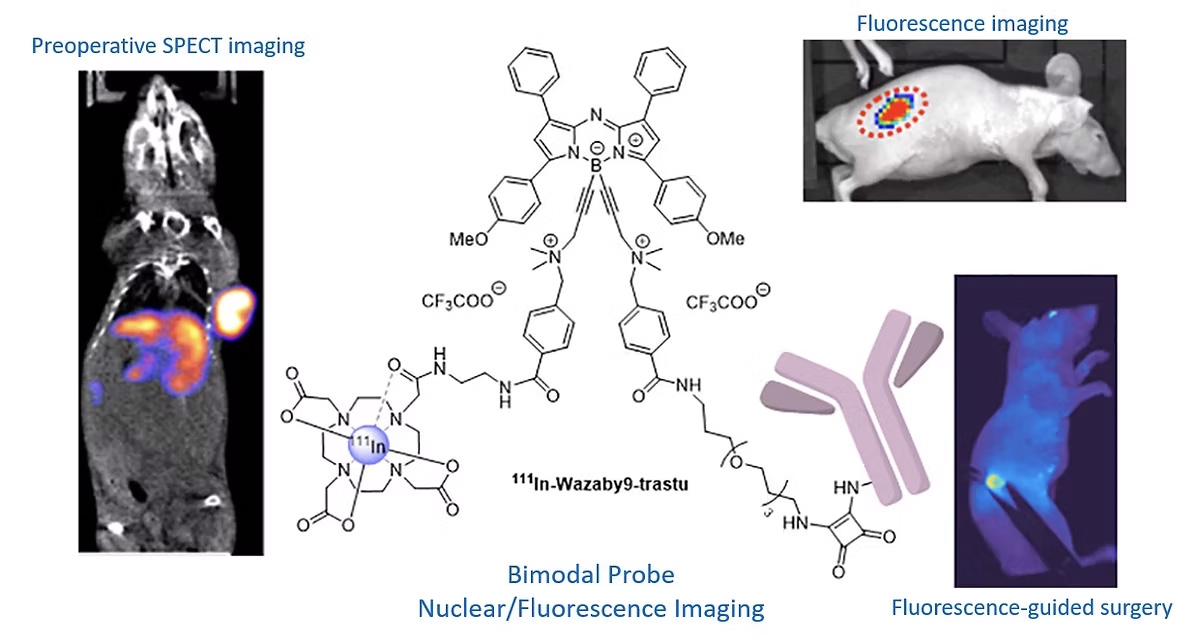
SOLID TUMOUR – FLUORESCENCE-GUIDED SURGERY
Despite the emergence of targeted therapies, surgery still plays an essential role in the treatment of colorectal and pancreatic cancers. A major problem is the difficulty of detecting and visualising tumour margins. Fluorescence-guided surgery (FGS) allows surgeons to better visualise anatomical structures in real time. In addition, FGS can be improved by combining pre-operative imaging to locate tumours accurately. In this project, we propose to design and evaluate bimodal imaging conjugates (nuclear imaging/fluorescence) to improve pre-operative staging (SPECT), intra-operative tumour detection and resection (FGS) and post-operative follow-up (SPECT). Our probes will be tested for FGS in various preclinical models mimicking human pathology, such as orthotopic models of colon and pancreatic cancer, but also models mimicking the formation of liver metastases or peritoneal carcinomatosis. The expected results of this project are fine visualisation of tumours before surgery (SPECT imaging) and more accurate detection by fluorescence, enabling the extent of resection to be minimised. Finally, it will be possible to detect the absence of recurrence using this compound a few weeks after surgery (SPECT).
This project has enabled our platform to contribute to a project funded by the INCA (PLBIO23-023) to the tune of 593,460 euros over 3 years (2024-2027) led by Dr Catherine Paul (École Pratique des Hautes Études – Université PSL) and in collaboration with the ICMUB (Dr Goze Christine) and Besançon University Hospital (Dr Doussot Alexandre).
Associated publications:
M. Privat, P.S. Bellaye, Chazeau E, Racoeur C, Adumeau P, Vivier D, Bernhard C, Moreau M, Collin B, Bettaieb A, Denat F, Bodio E, Paul C, Goze C. First Comparison Study of the In Vitro and In Vivo Properties of a Randomly and Site-Specifically Conjugated SPECT/NIRF Monomolecular Multimodal Imaging Probe (MOMIP) Based on an aza-BODIPY Fluorophore. Bioconjug Chem 2023.
M. Privat, P. S. Bellaye, R. Lescure, A. Massot, O. Baffroy, M. Moreau, C. Racoeur, G. Marcion, F. Denat, A. Bettaieb, B. Collin, E. Bodio, C. Paul, C. Goze, Development of an Easily Bioconjugatable Water-Soluble Single-Photon Emission-Computed Tomography/Optical Imaging Bimodal Imaging Probe Based on the aza-BODIPY Fluorophore. J Med Chem, (2021).
SOLID TUMOUR – TRIPLE NEGATIVE BREAST CANCER
Among the different types of breast cancer, triple-negative breast cancer is made up of tumour cells that do not express the receptors targeted by most of the innovative therapies currently available for other types of breast cancer. This cancer is particularly aggressive and associated with a high mortality rate, with a 5-year survival rate of less than 25%. Treating it remains a real challenge, as no targeted therapy is currently available. Triple-negative breast cancer is characterised by an infiltration of immunosuppressive cells which, by suppressing anti-tumour immune responses, promote tumour progression and the development of metastases. The aim of our project is therefore to develop new drugs targeting these immunosuppressive cells and to validate markers for therapeutic monitoring of such treatments.
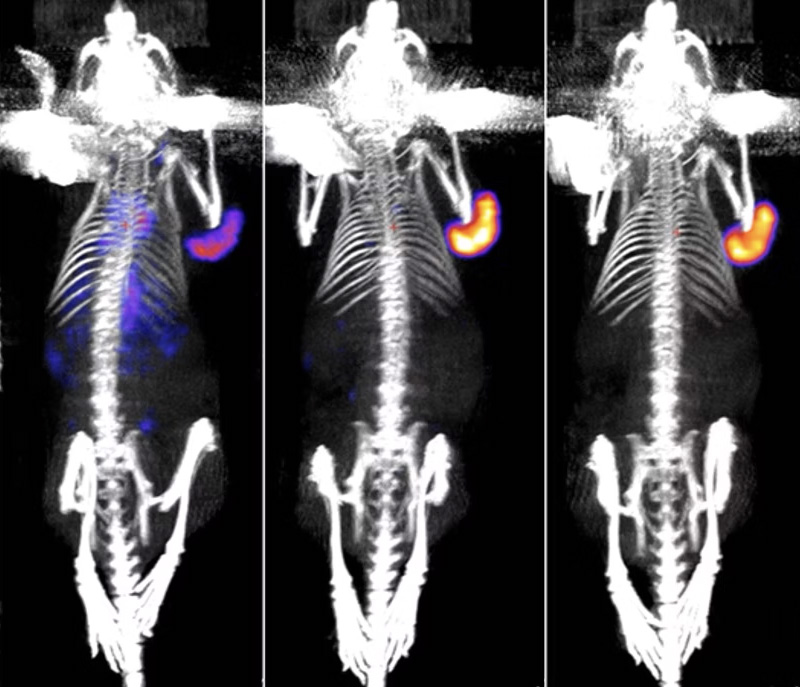
Our team is currently working in collaboration with the INSERM HSP-pathies team led by Dr Carmen Garrido on a stress protein, Gp96, whose increased presence in certain tumours is associated with a poor prognosis. We have shown that Gp96 is present in the membrane of a type of immunosuppressive macrophages: M2 macrophages, whose expression is correlated with a poor prognosis. We were able to image these immunosuppressive macrophages in vivo using SPECT imaging in our triple-negative breast cancer models and to monitor their reduction when a Gp96 inhibitor was used. Imaging these immunosuppressive macrophages could therefore be an innovative tool as a potential marker for prognosis, therapeutic prediction and/or follow-up in triple-negative breast cancer.
The results obtained in our preclinical model will provide a better understanding of the role of Gp96 in tumour progression and, we hope, open up a new avenue of targeted anti-Gp96 therapy with at least one Gp96 inhibitor, alone or in combination with existing anti-cancer treatments. They should also make it possible to validate the relevance of immunosuppressive macrophage detection by imaging to provide real-time information on their infiltration into the microenvironment around the tumour as predictive markers and for monitoring response to treatment in triple-negative breast cancer
SPECT imaging of M2 macrophages

Associated publications:
HEMATOLOGY – LYMPHOMA / MYELOMA
Our haematology projects aim to develop new imaging agents to better detect these cancers and offer solutions to facilitate patient follow-up and therapeutic decisions. Our research focuses on 2 main targets: the IL-1RAP protein, involved in inflammation and tumour development, is found to be overexpressed in several cancers, in particular acute and chronic myeloid leukaemia. And the CD38 receptor, currently used clinically in the treatment of myeloma, and anti-CD38 antibodies (e.g. Daratumumab) could also prove effective in certain forms of lymphoma, such as Burkitt’s lymphoma, which mainly affects children and young adults.
Haematological cancers or “blood cancers” affect around 35,000 people every year in France, mainly children, young adults and the elderly. Leukaemia, lymphoma and myeloma are the most common. Haematological cancers are caused by a malfunction in the maturation of blood cells: white blood cells, red blood cells and platelets, which leads to their abnormal proliferation. Blood cells first develop in the bone marrow, at the heart of the bones, before being released into the blood. The accumulation of altered blood cells slows down the normal functioning of other cells (fighting infections, preventing bleeding, etc.). The earlier the alterations appear in the maturation process, the more aggressive the cancer is likely to be. The discovery of new early biomarkers of progression and treatment efficacy in this type of pathology is crucial to improving patient care.
In this context, we are working in collaboration with Dr Christophe Ferrand (CanCell Therapeutics, Besançon) to develop a companion imaging biomarker for a new CAR-T cell therapy targeting IL-1RAP. This innovative therapy is currently in the clinical trial phase (Besançon University Hospital, CanCell Therapeutics) and a companion imaging biomarker targeting IL-1RAP could make it possible to better select patients with overexpression of this target and for whom this therapy is most likely to work. Our aim is therefore to develop a diagnostic companion based on an antibody targeting IL-1RAP modified so that it can be visualised by medical imaging by incorporating a radioactive isotope visible by PET/CT, SPECT/CT and/or PET/MRI imaging. Using this technology, it is also possible to replace the imaging radioactive isotope with another, more irradiating isotope, making it possible to obtain a therapeutic agent using the technique of targeted internal radiotherapy to specifically eliminate tumour cells.
MYELOMA
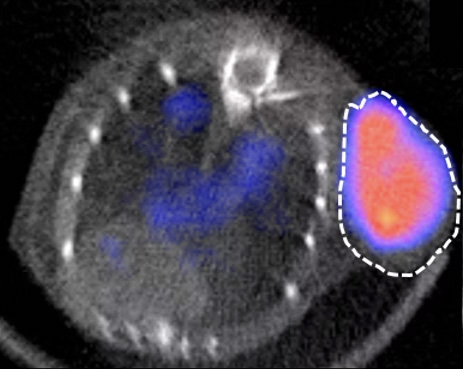
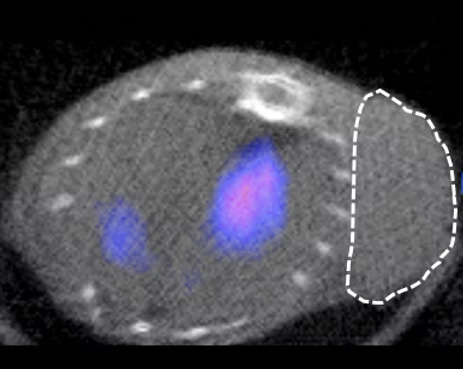
In order to demonstrate the usefulness of targeting the CD38 receptor in lymphomas, we are currently testing anti-CD38 antibodies in various lymphoma models in collaboration with the haematology department of Dijon University Hospital (Drs F. Girodon and C. Rossi). To do this, we are modifying these antibodies slightly so that they can be observed using medical imaging techniques (PET/CT, SPECT/CT, PET/MRI). In this context, it is also possible to replace the imaging radioactive isotope with another, more irradiating isotope, thereby making it possible to obtain a therapeutic agent using the technique of vectorised internal radiotherapy to specifically eliminate tumour cells.
LYMPHOMA
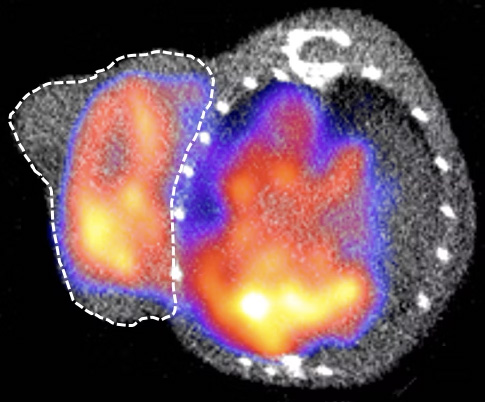
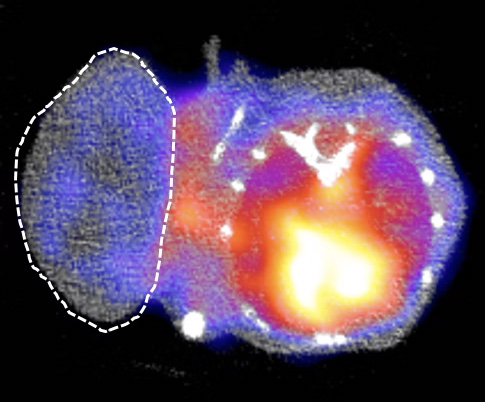
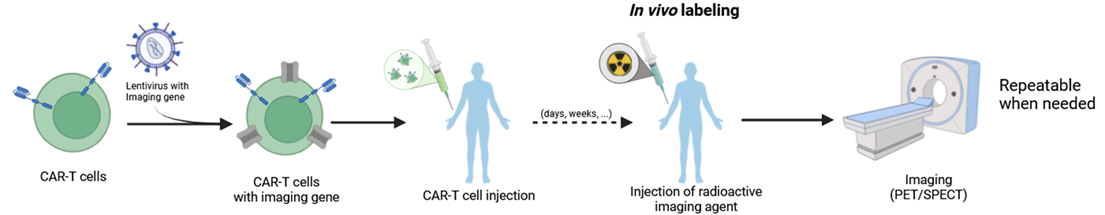
IMAGING OF CELL THERAPIES (e.g. CAR-T cells)
Cell therapy is an innovative treatment for a number of diseases, including cancer, heart disease and autoimmune disorders. Cell therapy involves either producing cells from a healthy donor in a laboratory or taking cells from a patient, then modifying them to give them a specific function, and re-implanting them into the patient. These therapies are often highly effective in certain patients, but are sometimes accompanied by adverse effects that can be serious. It is in this context that our research projects aim to give these therapeutic cells imaging capabilities so that they can be monitored in real time once they have been reinjected into the patient.
The objective of this project is to enable in vivo monitoring of CAR-T cell therapies using nuclear medical imaging techniques (SPECT/CT and PET/MRI).
CAR-T cells are T lymphocytes that have been genetically modified to express a CAR receptor capable of recognizing tumor cells. To track them within the patient’s body during treatment, we add a second reporter gene to the CAR-T cell genome so they express a protein that can trap certain nuclear imaging agents inside the cell. The imaging agent can then be injected at various points during the treatment in order to locate the CAR-T cells in the patient’s body. This could help assess the treatment efficacy and anticipate the onset of side effects.
At the same time, work is being carried out on the type of T lymphocytes used for CAR-T therapy using flow cytometry. The goal is to determine whether using a specific population of T cells can lead to improved treatment efficacy, particularly by generating CAR-T cells that persist longer.
This project is driven in collaboration with INSERM U1231, HSP-pathies team, Dr Carmen Garrido.